how to separate water and oil mixture Separate mixture brainly containing labelled separating funnel
Have you ever wondered how to separate a mixture of oil and water? It may seem like an impossible task since oil and water are known to be immiscible, meaning they cannot mix together. Well, there are actually a few ways to separate the two substances, and I’m here to explain it all to you! First up, let’s talk about the most common method: gravity separation. This method takes advantage of the fact that oil is less dense than water. When you pour the mixture into a container, the oil will naturally float to the top while the water stays at the bottom. The best way to do this is to use a separatory funnel, which allows you to pour off the oil layer from the top. This process can take some time, especially if the mixture contains a lot of oil, but it’s a simple and effective way to separate the two substances. If you’re dealing with a particularly stubborn mixture, you may need to use a centrifuge. A centrifuge is a machine that spins the mixture at high speeds, causing the oil to separate from the water due to the centrifugal force. Once the separation is complete, you can easily pour off the oil layer from the top. This method is commonly used in industrial settings and can be expensive, but it’s very effective. Another method is solvent extraction, which involves adding a solvent to the mixture that dissolves the oil. The solvent is then separated from the rest of the mixture and the oil is recovered by evaporating the solvent. This method may be necessary if the oil and water are too closely mixed for gravity separation or centrifugation to work. Now that we’ve covered the ways to separate the mixture, let’s take a closer look at the images provided. In the first image, we see a simple diagram of the gravity separation method. The “oil” layer is clearly labeled and the image shows the two layers slowly separating from each other. The image also provides a helpful tip to speed up the process by chilling the mixture beforehand, as colder temperatures can cause the oil to solidify and separate more easily. In the second image, we see a more detailed diagram of the solvent extraction method. The image shows the solvent dissolving the oil and the resulting mixture being separated in a separatory funnel. The “extract” layer is clearly labeled, as is the water layer that remains at the bottom. The image also provides a step-by-step process for recovering the oil using evaporation. In conclusion, separating a mixture of oil and water is a simple yet important task that can be accomplished using a few different methods. Whether you opt for gravity separation, centrifugation, or solvent extraction, just be sure to take your time and follow the proper steps for the best results.
If you are searching about How will you separate oil and water from their mixture? - Chemistry Q&A you’ve came to the right page. We have 5 Pics about How will you separate oil and water from their mixture? - Chemistry Q&A like science chemistry miscibility | Fundamental Photographs - The Art of Science, Draw a labelled diagram to show how you will separate the mixture containing oil and water and also science chemistry miscibility | Fundamental Photographs - The Art of Science. Read more:
How Will You Separate Oil And Water From Their Mixture? - Chemistry Q&A
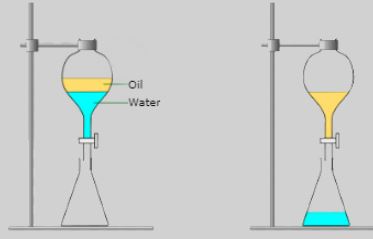 byjus.comseperating
byjus.comseperating
6th Grade And Onward!: Decantation
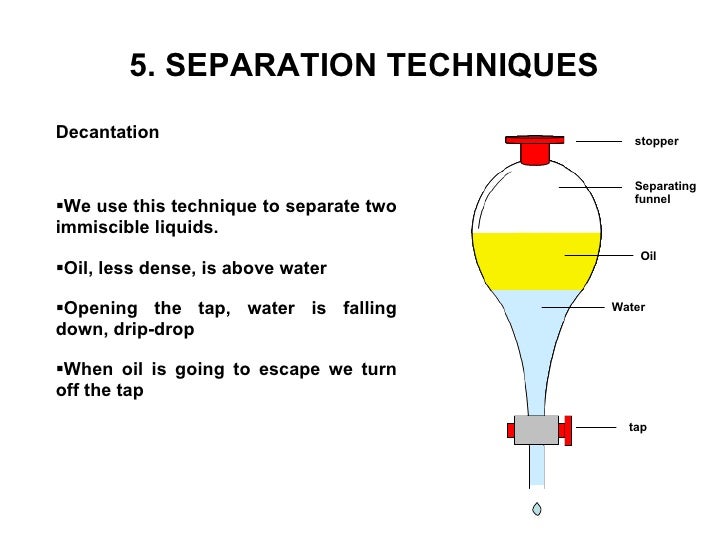 5thgrademiguelhernandez.blogspot.comwater oil separating decantation different methods liquids grade separate liquid sand densities 6th two used substances solid std6 onward smita
5thgrademiguelhernandez.blogspot.comwater oil separating decantation different methods liquids grade separate liquid sand densities 6th two used substances solid std6 onward smita
Science Chemistry Miscibility | Fundamental Photographs - The Art Of Science
 fphoto.photoshelter.comDraw A Labelled Diagram To Show How You Will Separate The Mixture Containing Oil And Water
fphoto.photoshelter.comDraw A Labelled Diagram To Show How You Will Separate The Mixture Containing Oil And Water
 brainly.inseparate mixture brainly containing labelled separating funnel
brainly.inseparate mixture brainly containing labelled separating funnel
How Do You Separate A Mixture Of Oil And Water? | Tips N’ Tutorials
 www.tipsntutorials.comoil mixture
www.tipsntutorials.comoil mixture
Oil mixture. Draw a labelled diagram to show how you will separate the mixture containing oil and water. Water oil separating decantation different methods liquids grade separate liquid sand densities 6th two used substances solid std6 onward smita